Promotional Features
Gut-heart axis: Study shows polyphenol-rich extracts can modulate the gut microbiota
Evidence suggesting the role of the gut microbiota in maintaining host health has become mainstream, supported by multiple strategies including probiotics, prebiotics, synbiotics or faecal microbiota Transplant to modulate the gut microbiome composition.
Among the list of prebiotic fibres are plant polyphenols which contain an estimated 90-95% that are not absorbed in the small intestine but reach the colon, where they undergo extensive biotransformation by the gut microbiota. Dr. Robert E. Steinert, main author of the study explained that “natural products rich in polyphenols have been hypothesized to lower plasma trimethylamine-n-oxide (TMAO), an emerging biomarker known for its proatherogenic effects by beneficially modulating the intestinal microbiota”.
Dr. Marcus Claesson, the partner overseeing the microbiome analysis and bioinformatics, added: “Previously thought to be a waste product of choline metabolism with no physiological function, trimethylamine-N-oxide (TMAO), a chemical metabolite derived from diet and metabolized by the gut microbiota, is now supported by growing evidence for its involvement in inflammation, cardiovascular disease, and obesity.
“A high level of TMAO may increase the risk of clot-related events like heart attack and stroke, according to research.1 Thus, a changed gut microbiota and increased intestinal permeability may be the causes of chronic inflammation, which worsens cardiac function.2
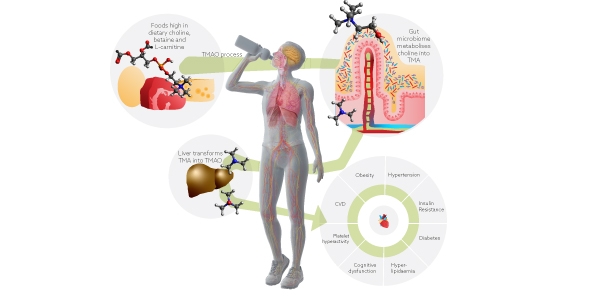
TMA is a microbial metabolite produced by the gut microbiome from dietary phosphatidylcholine and L-carnitine, commonly found in red meat, cheese and eggs. When absorbed by the intestinal epithelium and transported to the liver, TMA is transformed into TMAO. TMAO is known for its proinflammatory and proatherogenic activities. Higher levels of TMAO have been linked to major adverse cardiovascular events.
As TMAO is dependent on metabolism by the intestinal microbiota, gut microbiome strategies have emerged for the prevention of cardiovascular disease. The focus is on natural products, due to the side effects and the resistance potential limits of antibiotic strategies. The perfect candidate would be a natural solution which might be able to inhibit microbial TMA production. Prebiotics seem to be the right candidate as they are a substrate that is utilized by the host microorganisms conferring a health benefit.
About this study
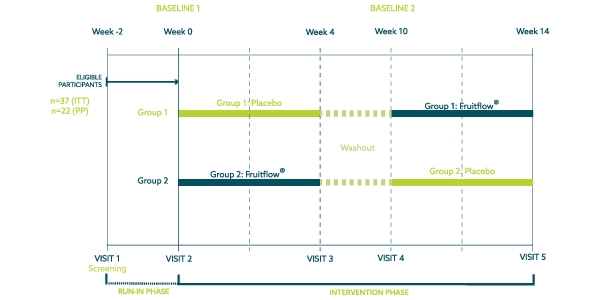
A randomized double-blind, placebo-controlled crossover study was conducted in order to assess the research question. Consisting of five visits and six-week washout, the study lasted 14 weeks after a 21-day run-in period.
The study population consisted of 40 healthy, overweight, and obese adults (BMI 28-35 kg/m2), aged 35-65 years. Of these, 20 participants were allocated to the Placebo/Fruitflow® arm (group 1), and 20 participants to the Fruitflow®/Placebo arm (group 2). Three participants self-withdrew after randomisation, the remaining 37 participants completed the study as planned and analysed in the Intention-to-Treat population.
All participants were evaluated on a case-by-case basis by an independent committee before unblinding of the data to determine inclusion to the per-protocol population. 15 subjects were excluded from the Per Protocol subset analysis due to intake of antibiotic concomitant medications and/or product intake compliance below 80% which would impact the efficacy assessment of microbiome outcomes. Blood, urine and faecal samples were collected in this study. A venous blood sample was collected for safety profiling and analysis of plasma TMAO and lipopolysaccharides (LPS). Urine sample was collected for analysis of urine TMAO. Faecal samples were utilised to analyse the microbiome composition.
In addition to biological samples, questionnaires such a Gastrointestinal Symptom Rating Scale (GSRS) and Bristol Stool Chart were completed to assess tolerability. A Food Frequency Questionnaire (FFQ) was completed by the participants to determine stability of dietary intake over time.
In addition to the main intervention, a subgroup of nine participants completed an acute egg challenge. They were instructed to follow a standardised diet consisting of a high-yolk scrambled egg recipe. The goal of this challenge test was to determine the effect of intervention on postprandial plasma and urine TMAO concentrations.
The investigational product, Fruitflow®, was commercially produced by DSM Nutritional Products, Basel, Switzerland, in powder format, then encapsulated for the purpose of this trial. Maltodextrin was used as a placebo control.
The results
Fruitflow® significantly decreased fasting blood and urine TMAO when compared to baseline as well as plasma LPS, a marker of intestinal permeability and low-grade inflammation. Moreover, when compared to the placebo group, this was also significantly different for urine TMAO. Dr. Steinert further explained the mechanism of action studied in this trial: “Indeed, Fruitflow® seems to modulate the gut microbiota in a way that resulted in a lowering of urine and plasma TMAO.
“Moreover, we found that Fruitflow® reduced plasma lipopolysaccharides (LPS), a gut microbiota-derived factor that has been suggested to drive onset and progression of chronic inflammation-related diseases such as obesity, type 2 diabetes or non-alcoholic fatty liver disease (NAFLD).”
As mentioned by the authors, a clear distinction was observed also between plasma samples when applying untargeted metabolomics, with TMAO being the top-ranking feature driving the differences found across the Fruitflow® product group compared to the control group. Significant changes in microbial beta diversity were also highlighted.
In addition, the microbiome composition changed significantly in the Fruitflow® product group. Lower levels of Bacteroides, Ruminococccus and Hungatella were found, which are known for the involvement in TMA/TMAO metabolism. Dr. Steinert developed on the framework of these results within the overall gut-heart axis research, adding “…certainly, these results support earlier findings that polyphenol-rich extracts can lower TMAO concentrations and that this may be related to a targeted beneficial modulation of the gut microbiota.”
Dr. Claesson added: “We also noted a significant decrease in Rumonococcus faecis and Hungatella hathewayi within the Fruitflow® product group. It is crucial to remember that Ruminococcus was discovered to have a positive correlation with plasma TMAO levels in a study on rats, while Hungatella hathewayi is known as a TMA producer.”
The findings of this study are in line with the studies showing a reduction in plasma TMAO suggesting reproducible effects with polyphenol rich extracts. As a conclusion of this trial it was asserted that polyphenol-rich extracts can modulate the gut microbiota conferring a host health benefit.
Future perspectives
The fact that TMAO was the main component explaining the differences in plasma samples, places this metabolite as a robust biomarker for cardiovascular disease. Dr. Claesson said: “Findings in this study provide support to the association between higher levels of TMAO and fecal microbiota. In this study, we identified potential TMA-producing bacteria that could be used as a prognostic biomarker or as a therapeutic target in patients with several cardiovascular conditions.”
In addition, plasma TMAO levels could be relevant to monitor inflammation and age‐related cognitive dysfunction. The study suggests intervention aiming to decrease plasma TMAO, such as polyphenol extracts, may reflect novel therapeutic option for neurodegenerative diseases as well as cardiovascular disease. Dr. Steinert elaborated on the potential of novel dietary solutions targeting TMAO by suggesting it could “open the way to develop novel dietary solution for cardiometabolic benefits but also benefits along the gut-brain axis” and “suggests a role that TMAO levels may also be relevant for monitoring (age-related) inflammation and cognitive dysfunction”.
This trial presented positive results, with the main hypothesis of the research confirmed: that four weeks of supplementation with Fruitflow®, a water-soluble tomato concentrate, rich in secondary metabolites including polyphenols, decreases fasting blood and urine TMAO when compared to baseline as well as plasma LPS, a marker of intestinal permeability and low-grade inflammation. Extending on the existing approved EC Article 13.5 health claim.
The study opens a door to future research on this field, as reinforced by Dr. Steinert: “It is exciting to see a beneficial role of Fruitflow® also on the gut microbiome. We will be developing dietary supplement solutions targeting the gut-heart and gut-brain axis.”
References
1. Steinert R.E.; Rehman A.; Tyree S.M. et al. A water-soluble tomato extract rich in secondary plant metabolites lowers trimethylamine-n-oxide and modulates gut microbiota: a randomized, double-blind, placebo-controlled cross-over study in overweight and obese adults. The Journal of Nutrition, Volume 153, Issue 1, 2023, Pages 96-105, ISSN 0022-3166.
2. Zhu W.; Gregory JC.; Org E.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016 Mar 24;165(1):111-124. doi: 10.1016/j.cell.2016.02.011. Epub 2016 Mar 10. PMID: 26972052; PMCID: PMC4862743.
3. Sandek A.; Bauditz J.; Swidsinski A.; et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007 Oct 16;50(16):1561-9. doi: 10.1016/j.jacc.2007.07.016. Epub 2007 Oct 1. PMID: 17936155.
Fruitflow® is a trademark of Provexis, plc.